ISB Researchers Show Genetic Risk for Disease Often Reflected in Our Blood
 isbscience.org/news/2020/08/17/isb-researchers-show-genetic-risk-for-disease-often-reflected-in-our-blood/
isbscience.org/news/2020/08/17/isb-researchers-show-genetic-risk-for-disease-often-reflected-in-our-blood/
Artistic depiction of an individual’s genetic risk for disease being “reflected.” © ISB
Humans do not develop disease overnight. Rather, diseases develop gradually over years — sometimes decades — before symptoms appear, and are due to malfunctioning physiological processes brought about by our genes and environment.
In research just published in the journal Proceedings of the National Academy of Sciences (PNAS), scientists from Institute for Systems Biology (ISB) and partner institutions have shown how an individual’s genetic risk for disease is often reflected in their blood.
“A barrier to this area of research is that you can’t usually access the parts of the body where the disease actually starts to develop,” said Dr. Michael Wainberg, first author of the study. “You’re not exactly going to drill into the skull of someone at risk for depression just to take a brain tissue sample! We used blood as a proxy for what’s going on in the rest of the body.”
Researchers studied blood analyte measurements of nearly 5,000 people and found that those with higher genetic risk for each of several dozen diseases have hundreds of detectable changes in protein levels, metabolite levels, and clinical lab tests. Researchers also found that the changes were frequently present in people with the disease, suggesting the changes represent early warning signs of disease that occur before symptoms appear. Some of these changes directly suggest therapeutic strategies and influences on longevity.
“Monitoring how genetic risk manifests in the blood provides intriguing clues to how we might be able to intervene in avoiding disease before symptoms arise,” said Dr. Nathan Price, co-corresponding author on the paper. “Developing such capabilities is central to the idea of scientific wellness that we have been pursuing at ISB for several years.”
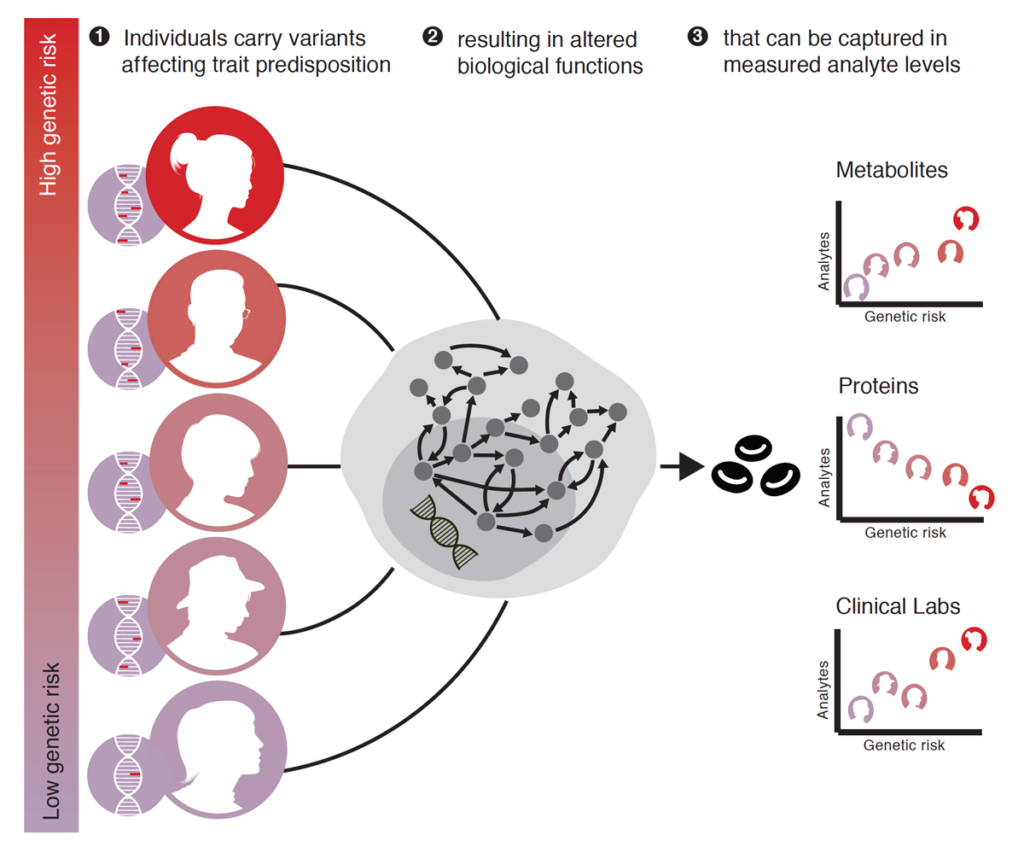
Study Figure: Conceptual overview
The work is inspired by P4 medicine — an approach pioneered by ISB Co-founder Dr. Lee Hood that is predictive, preventive, personalized, and participatory.
“The holy grail of medicine is identifying and preventing disease long before it manifests. While this remains an aspirational goal, we have made great strides in just the past few years, and this research takes us a step closer,” said Hood, co-corresponding author of the study.
This research builds on other important ISB findings, notably that molecular and physiological information can determine an individual’s biological age, which can serve as a more effective and reliable predictor of overall health than chronological age; that the alpha diversity of an individual’s gut microbiome can be accurately predicted by examining metabolites in the blood; and that combining personal, dense, dynamic data (PD3) clouds with tailored behavioral coaching can optimize wellness and the PD3 clouds can identify early transitions into disease states and facilitate the reversal of some disease states back to wellness.





