Systems Biology and the Environment at a Glance:
- Systems biology helps ISB scientists better understand how microbes interact with ecosystems.
- Systems biology can help scientists understand how microorganisms can clean up environmental waste and create biofuels.
- Systems biology can lead to predictions about how to make targeted changes to microbial networks to increase sustainability.
Systems Biology and the Environment Explained
Achieving environmental sustainability is one of civilization’s historic challenges. Since our inception, ISB has been using systems science to improve the understanding of the interactions of microbes and ecosystems and create a new generation of sustainable tools and strategies that can be deployed to address this challenge.
This systems understanding is essential to explain and predict consequences of complex phenomena such as climate change, so that responsible and sustainable strategies for addressing these real-world issues can be developed.
Interestingly, the solutions to these forward-looking questions lie in our past. Over 3.5 billion years of evolution, for example, biological systems have scanned every inch of our planet to solve some of the most complex environmental problems. We have discovered life on ocean floors under enormous pressure where temperatures are above boiling; in halite crystals 250 million years old; under conditions where sulfur instead of oxygen is used for respiration; and in arctic glaciers where temperatures are well below freezing.
Herein may be solutions to some of our own environmental challenges. Using systems biology approaches, we may be able to recombine various mechanisms within these diverse organisms to deal with some extraordinary human problems. Modern sequencing technologies are cranking out genome sequences for diverse organisms by the hundreds, if not thousands. Computational approaches to decode these genomes are also advancing rapidly. These technologies, while identifying the constituent parts lists of genes, RNAs, and proteins, do not explain why organisms with similar parts can solve very different problems. For this, a systems approach is necessary. Such approaches can characterize how unique biological circuits within each of these organisms assemble dynamically through environment-dependent synthesis, sharing and re-use of constituent parts.
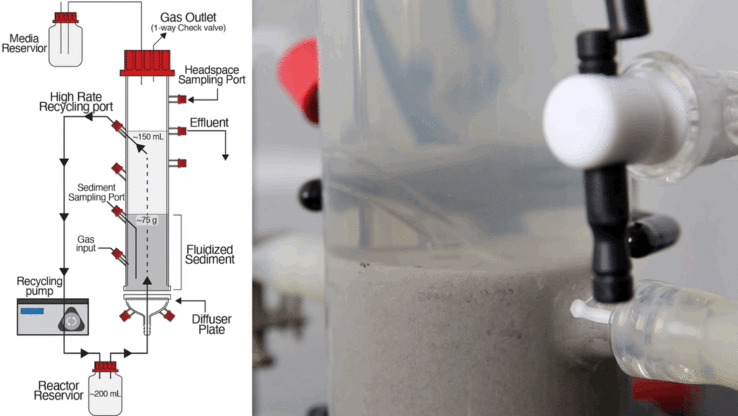
By applying sophisticated technologies for cell culturing, perturbation and high-resolution measurement of responses, ISB researchers have mapped at the molecular level biological components and function of organisms found in extreme environments. Using rapidly advancing synthetic biology approaches, we are learning how to precisely re-engineer this exquisite biological circuitry to obtain desirable properties. More importantly, by plugging the re-engineered circuit back into the model of the organism as a whole, we can identify and make compensatory changes elsewhere in the organism’s overall cellular network to restore general systems fitness.
This powerful approach with bacteria, being studied at ISB, could potentially provide sustainable solutions for some very pressing human problems, such as cleaning up radionuclide waste in holding tanks by re-engineering metal-reducing organisms to grow under high salt, high temperature, and high radiation conditions.
Why Systems Biology
When ISB scientists take on a challenge, they look at its components and figure out the best solution not based on what strategies already exist but what the problem demands.
Why Model Organisms
When an organism’s environment changes, it responds by altering the activity of networks of genes. A major goal of systems biology is to understand these networks and their interactions with environmental factors in sufficient detail to predict an organism’s molecular-level response to changing conditions. The Nitin Baliga Lab at ISB is pursuing this goal using an unusual group of model organisms: the Archaea. An ancient group of single-celled microorganisms that may comprise up to 20 percent of the world’s biomass, the Archaea have relatively compact and easily manipulated genomes. Also, many archaeal species have evolved biological systems that enable them to live in extreme conditions such as hot springs or salt lakes.



 isbscience.org/research/environment/
isbscience.org/research/environment/