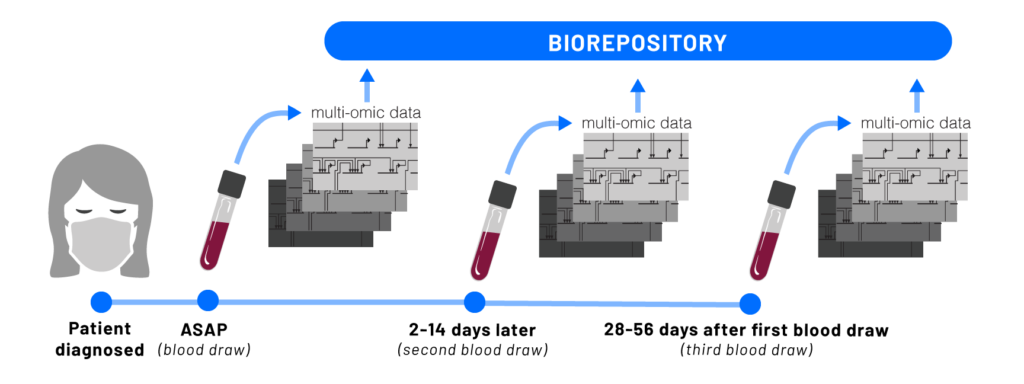
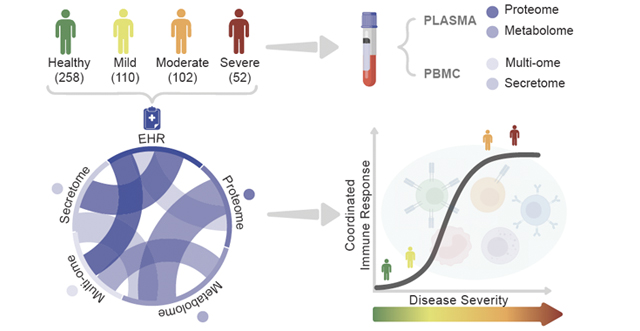
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease has spread globally since late 2019, resulting in the 2019-20 coronavirus pandemic. Common symptoms include fever, cough, and shortness of breath. While the majority of cases result in mild symptoms, some patients progress to pneumonia and multi-organ failure. The death rate is estimated at between 1% and 5% but varies by age and other health conditions. Illustration of the process of infection and immune response in patients. Once a patient is infected with SARS-CoV-2, the body begins creating unique protective molecules that can be studied to understand and treat the disease. Seattle’s scientific and research community understands the urgency of this pandemic. It has embraced collaboration for the greater good, shared research findings to expedite knowledge and the development of therapies and vaccines, and has risen to the mission of using all of its knowledge and tools to better understand, and ultimately overcome, this challenge. ISB scientists were quick to tap into their research and medical networks to form collaborations to study the disease. In March, Dr. Jim Heath, president of ISB and Dr. Jason Goldman, an infectious disease physician at Swedish were the first to put their heads together to design a study that is now underway. In April, Merck and ISB announced a research collaboration that includes vital funding for the study. A full list of collaborative partners and funders is below.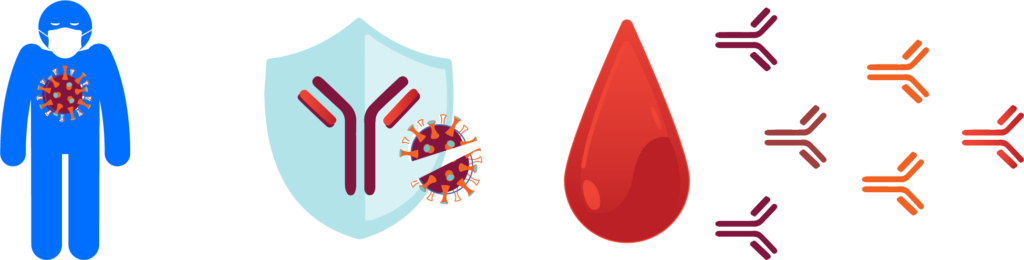
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.