Help Us Fight Coronavirus
You can support our groundbreaking COVID-19
research by making a contribution today.

 isbscience.org/news/2020/07/09/isb-builds-a-digital-platform-for-covid-19-research-study-and-beyond/
isbscience.org/news/2020/07/09/isb-builds-a-digital-platform-for-covid-19-research-study-and-beyond/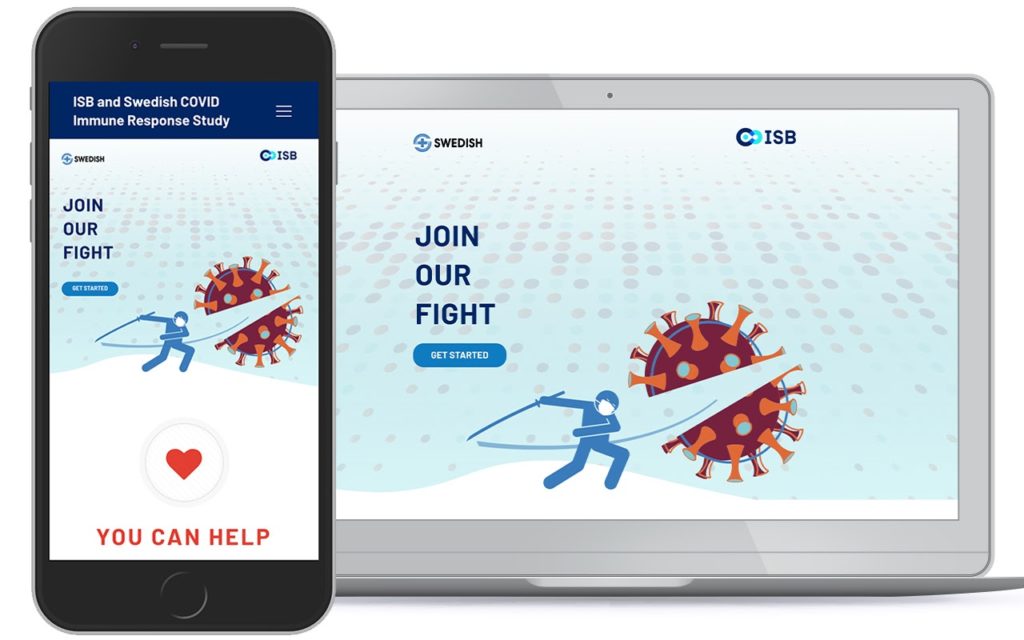
View of the responsive recruitment website and electronic consent platform as seen on mobile phone and desktop devices.
ISB is co-leading a one-of-a-kind COVID-19 research study to learn why those infected have drastically different outcomes and to identify targets for medicines and vaccines.
In a multi-institutional study of a highly infectious disease like COVID-19, paperless consent for study participants is critical. Oftentimes, participants need to enroll at home, rely on a proxy to consent for them due to severity of illness, and/or rely on mobile phones or devices to read and understand study details and agree to participate.
One component of the COVID-19 Immune Response Study is a recruitment website with an IRB-approved and HIPAA-compliant electronic consent platform for enrolling patients.
COVID-19-positive study participants give blood at three different stages during their infection (baseline, acute illness and convalescence). Patients have various levels of symptoms from mild to severe. Patient information is extracted from electronic medical records and multi-omic data is collected on all patients. Given these complexities, a digital platform was needed to manage the collection, analysis, and distribution of de-identified patient data to researchers.
The Health Data Science Lab at ISB was up to the task of converting an otherwise paperwork-heavy process into a digital platform.
“We are working with an experienced, professional team of trial coordinators and physicians at Swedish Medical Center,” said Andrew Magis, director of the Health Data Science Lab. “However, studies such as this one are a lot of work, and hospital staff are busy caring for a lot of sick people right now. We want to use technology where possible to reduce their workload and make the study run more efficiently.”
The digital platform serves many uses, including:
By creating an e-consent platform, it gives already overworked health care workers and hospital staff a digital solution to reduce workload and streamline the trial.
For example, a patient with mild symptoms may have their first blood draw at a Swedish Medical Center location, but after that, a mobile phlebotomist may come to their home to collect their sample. Having technology to support the complex logistical requirements of this study offers great benefits over the traditional paper-based methods.
Magis and his Health Data Science Lab colleagues are building this technology to be scalable, and applicable for other studies ISB will work on with Providence Health and other clinical partners.
“Ultimately we want to create a cloud-based system that interfaces with hospital clinicians, simplifies patient recruitment, blood draw scheduling and sample collection, automates data ingestion and sample biobanking, and enables de-identified multi-omic data to flow to ISB and our collaborators,” said Magis. “It’s an ambitious goal, but one that is possible with the right vision and motivation.”
ISB, Swedish Medical Center, Merck, and several other institutions are collaborating in the COVID-19 Immune Response Study to follow hundreds of patients who contract COVID-19 to learn why those infected have drastically different outcomes. Learn more about the research study here.
Category: Press, Press Release, Translational Medicine
Tags:Andrew Magis, cloud computing, COVID-19, digital platform, e-consent, Health Data Science Lab, HIPAA compliant, IRB approved, mobile friendly, Multi-omic, responsive, study, Swedish Medical Center, technology, technology development, translational medicine
You can support our groundbreaking COVID-19
research by making a contribution today.

New peer-reviewed research out of ISB highlights the strengths of large language models in uncovering social determinants of health while underscoring the need for human oversight and improved de-identification methods.

ISB Assistant Professor Dr. Sid Venkatesh is the co-first author of a paper in the journal Science. While at Washington University in St. Louis, Venkatesh and colleagues identified a novel gut microbial enzyme that impacts satiety-related signaling pathways in undernourished children treated with microbiota-directed complementary foods.

Faduma Hussein recently joined the ISB Education team as the Public Health Ambassador Coordinator, becoming only the fourth AmeriCorps member to serve at ISB. In this Q&A, she shares insights into her education, what drew her to ISB, career aspirations, and more.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.