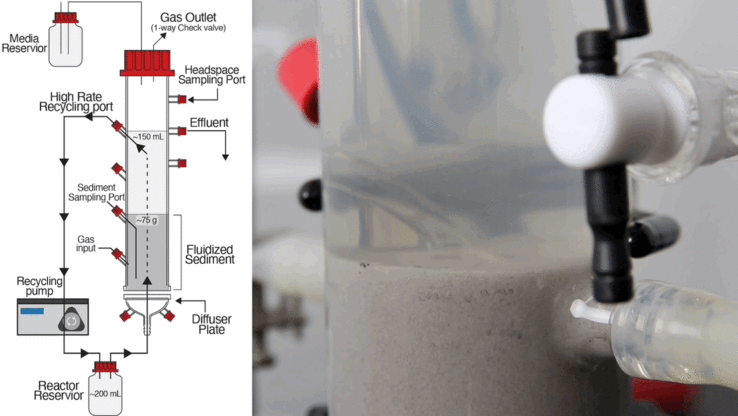
Sept. 25, 2014
3 Bullets:
- Novel systems approach uses high-resolution structures of protein-DNA complexes to predict where transcription factors (genetic switches) bind and regulate the genome.
- This approach can help researchers better understand and predict binding sites for non-model organisms or ‘exotic’ species.
- Having such insight and predictive capabilities is critical for reverse- and forward-engineering organisms that could be pivotal for new green biotechnologies.
By Jake Valenzuela and Justin Ashworth
Researchers at the Institute for Systems Biology (ISB) have developed a new method to predict the locations of genetic on-off switches (transcription factors) that is more powerful and accurate than other existing approaches. Having this predictive capability allows researchers to better understand the gene networks that control cellular physiology and adaptation in new or understudied organism species that may impact the development of green biotechnologies.
In the study, lead author, Justin Ashworth, and colleagues in the Baliga Lab at ISB, demonstrated the power of the new approach by accurately predicting how subtle changes in the DNA-recognition sequences within proteins has altered where they bind and regulate the genome of a salt-loving microbe, Halobacterium salinarum.
Transcription factors (TFs) are fundamental to gene regulation, binding to DNA and typically turning on or off from one to hundreds of genes. For non-model organisms, it is sometimes difficult to make predictions about gene regulation due to their novelty: not everything looks or functions like E. coli or any other long-studied laboratory organism. In many archaeal species, including H. salinarum, new and expanded families of transcription factors appear to regulate metabolism in ways that we have not seen before. In order to understand the ways that these TFs form gene regulatory networks and control cellular physiology, more powerful prediction methods are needed than existing gene lineage methods.
Our detailed understanding of the relationship between sequence, molecular structure, and function in protein and DNA coding can be used to improve the prediction of gene regulatory relationships and networks by explicitly considering the high-resolution three-dimensional structure of proteins and DNA, the energetic implications of evolutionary variations in proteins and DNA sequences can be more precisely estimated. In this paper, ISB researchers obtained greater power to predict the different DNA binding site preferences of eight Lrp-like feast/famine regulatory proteins (FFRPs) that were recently discovered in the H. salinarum genome. These FFRPs are distantly related to a transcription factor (Lrp) in E. coli, but sense different signals as well as regulate very different genes in archaea. Through a combination of structure-based predictions and experimental measurements, ISB researchers were able to infer that the archaeon H. salinarum utilizes an FFRP protein to coordinately regulate its carA and carB genes, whereas the same genes in E. coli are regulated collectively in an operon by a completely different transcription factor. Such distinctions can have enormous impacts on the gene regulatory networks of the organism.
Incorporating molecular and physics-based information into the prediction of gene regulatory networks is useful particularly in new species and non-model organisms, for which simple homology to well-studied organisms (e.g. E. coli) is insufficient to explain their biology.
The increased resolution and discriminatory power that this new method affords will be particularly useful for understanding non-model organisms found in new environments and ecosystems, as well as for reverse- and forward-engineering non-model organisms that are desirable for bioproduction processes. Understanding how genes are regulated and by which transcription factors is a critical aspect of understanding how cells function and adapt. These increased predictive capabilities will facilitate a systems-level understanding of non-model organisms, as well as the re-engineering of cellular control systems for new green biotechnologies.
Title: Structure-based inference of feast/famine regulatory targets
Publication: PLOS ONE
Authors: Justin Ashworth, Christopher L. Plaisier, Fang Yin Lo, David J. Reiss, Nitin S. Baliga
Link: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0107863



 isbscience.org/research/new-tool-uses-3-d-protein-dna-structures-to-predict-locations-of-genetic-on-off-switches/
isbscience.org/research/new-tool-uses-3-d-protein-dna-structures-to-predict-locations-of-genetic-on-off-switches/