Dec. 7, 2015
3 Bullets:
- Human activities are continually reshaping the global ecosystem. Of particular concern is the increasing level of atmospheric and dissolved carbon dioxide (CO2), arising in part, from our dependence on fossil fuels.
- Microalgae sequester carbon dioxide and direct it to the production of bio-oil in response to nutrient stress making them an ideal source for sustainable biofuel.
- Researchers at ISB have developed the first mechanistic and predictive gene regulatory network model for the green algae Chlamydomonas reinhardtii, while also extending and improving its metabolic network model.
By Jake Valenzuela, Saheed Imam and Adrian de Lomana
To the casual observer, algae may appear to be a nuisance. But for researchers, photosynthetic microalgae and other microbes have the potential to become sustainable biofactories that can economically produce renewable biofuels and a wide variety of other valuable commodities.
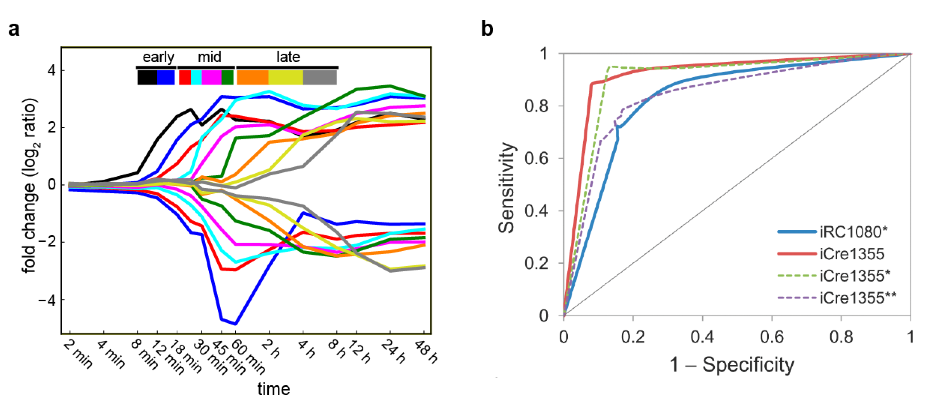
Computational Approaches to C. reinhardtii physiology. a. Differentially expressed transcripts are organized as a sequential set of transcriptional waves. b. ROC curves comparing the predictions of iCre1355 to other models for gene deletion phenotype data.
One such group of microalgae is Chlamydomonas reinhardtii – Chlamy for short. In an effort to better understand the gene regulatory and metabolic networks of this single-celled alga, researchers at Institute for Systems Biology studied the changes in Chlamy’s genetics and metabolism that cause them to capture and store carbon dioxide. Chlamy and other microalgae, under stressful conditions, will sequester carbon dioxide (CO2) in the form of lipids or “bio oil.” But to advance biofuel sustainability requires rational engineering of microalgae to increase their productivity, which can be achieved by reverse engineering of their metabolic and regulatory circuitry.
ISB researchers have long studied (see cMonkey article) the genetics and metabolic networks of microbes because of their ability to efficiently harness solar energy, sequester harmful CO2 and grow rapidly. The predicted consequences of CO2 released into the atmosphere as a result of human activities – such as global warming, climate change and ocean acidification – could be detrimental to both terrestrial and aquatic habitats. Photosynthetic organisms evolved over millions of years to consume CO2, utilize light energy and split water to produce oxygen. Indeed, they are responsible for the available oxygen in our atmosphere today.
In ISB’s work to elucidate the transcriptional and metabolic changes that drive lipid accumulation in C. reinhardtii, researchers found, strikingly, a transcriptional program organized into elegant sequential waves of transcription spanning from 12 minutes to 8 hours after nitrogen starvation. Further, they developed a revised and significantly improved genome-scale metabolic model for C. reinhardtii named iCre1355, which represents a major advance over previous models, both in content and predictive power. iCre1355 showed improved accuracy in prediction of growth rates, triacylglycerol production and the metabolic response to changing light availability.
Thus, iCre1355 should serve as a useful resource for studying the metabolic processes of this and related microalgae. Both models are publicly available. The gene regulatory network model is available as a community-wide web-resource at the Chlamy Network Portal. This platform provides a collection of tools that enable the rational prioritization of molecular targets for bioengineering microalgae, which will be of great benefit to the growing biofuel research community.
Read more about ISB’s work with Sustainable Biofuel Research at: Systems Biology and the Environment.
Journal: The Plant Journal
Title: A refined genome-scale reconstruction of Chlamydomonas metabolism provides a platform for systems-level analyses.
Authors: Saheed Imam, Sascha Schäuble, Jacob Valenzuela, Adrian L. G. de Lomana, Warren Carter, Nathan D. Price and Nitin S. Baliga.
Link: PubMedJournal: Biotechnology for Biofuels
Title: Transcriptional program for nitrogen starvation-induced lipid accumulation in Chlamydomonas reinhardtii
Authors: Adrian Lopez Garcia de Lomana, Sascha Schäuble, Jacob Valenzuela, Saheed Imam, Warren Carter, Damla D. Bilgin, Christopher B. Yohn, Serdar Turkarslan, David J. Reiss, Monica V. Orellana, Nathan D. Price and Nitin S. Baliga
Link: PubMed



 isbscience.org/research/microalgae-may-be-the-biofactories-of-a-sustainable-future/
isbscience.org/research/microalgae-may-be-the-biofactories-of-a-sustainable-future/