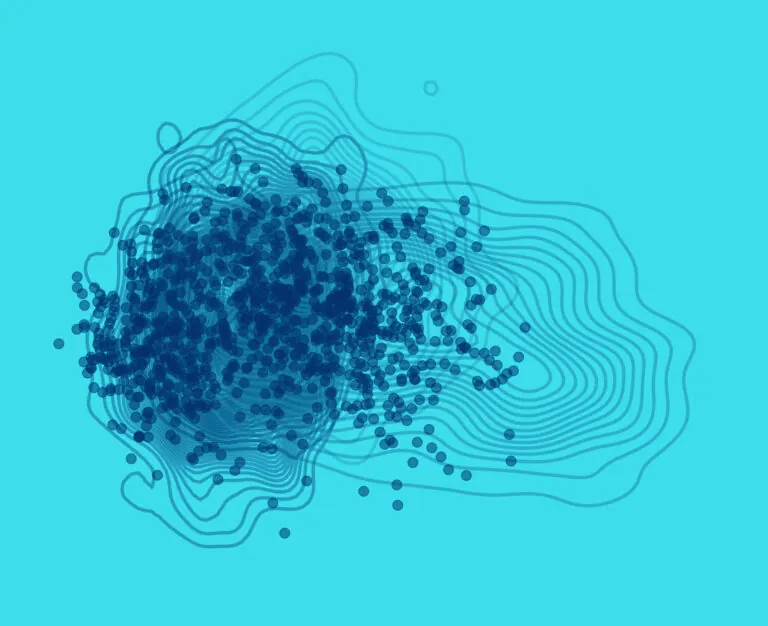
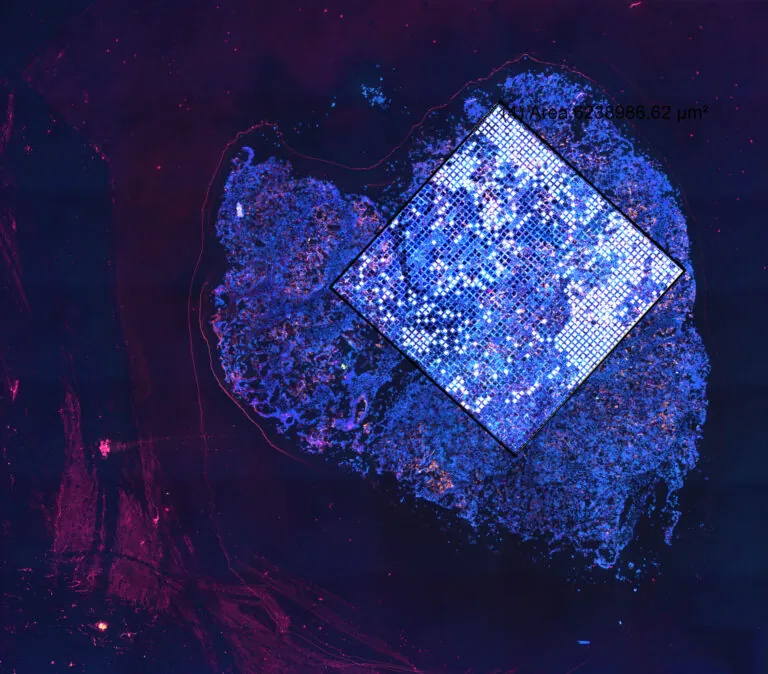
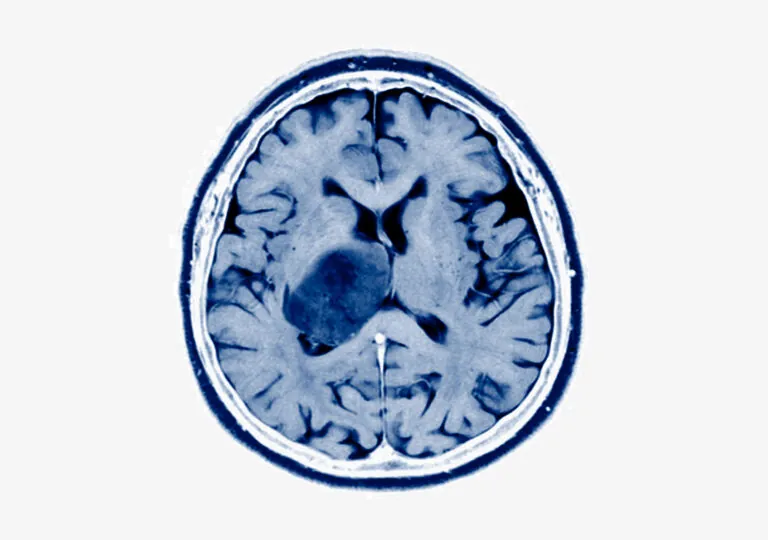
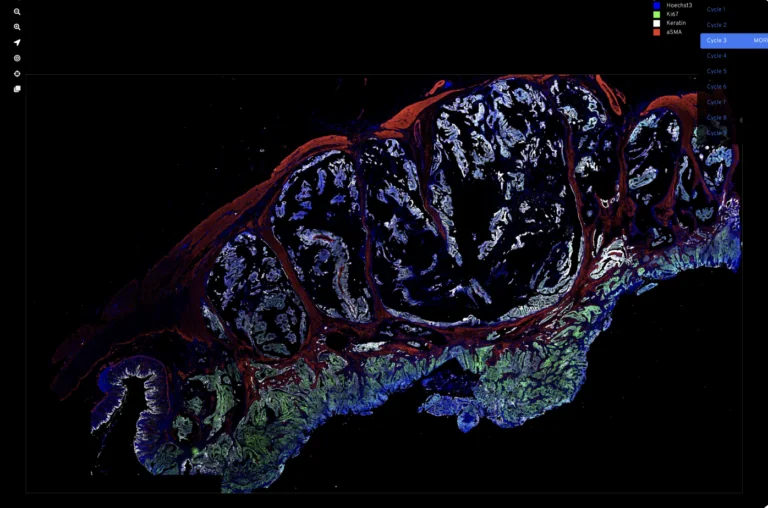
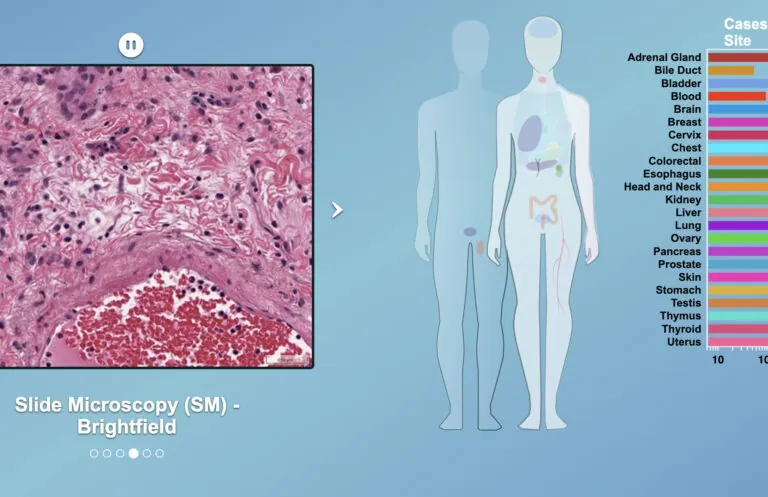
We work at the leading edge of cancer research.
What the world needs now is effective and accessible cancer therapy. That means the best treatment, at the optimum time, tailored to each patient, and available to all. To that end, we study tumor biology, develop early diagnostics, explore ways to maximize existing treatments, create even better treatments, and promote wider access to the most up-to-date data.