Exploring Longevity and Age-Related Disease
By 2034, US adults 65 and over are expected to outnumber children, making improved understanding of the molecular factors underpinning longevity, healthy aging and age-related disease of critical importance. The NIH’s Longevity Consortium – in which the Hood Lab’s Noa Rappaport co-leads three projects – aims to better understand these factors and identify possible pathways for drugs or other treatments.
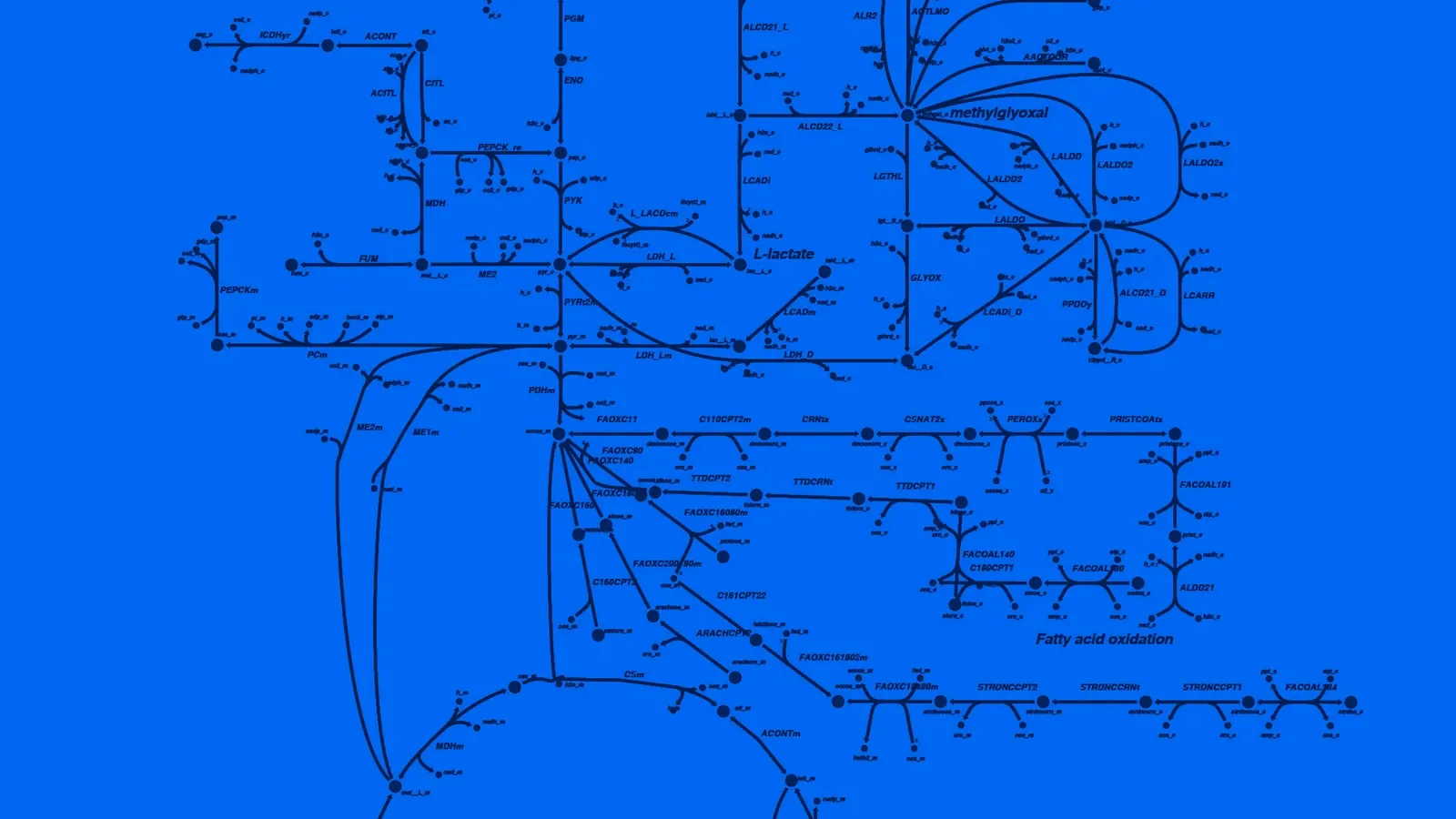
Image of a metabolic pathway from lead author Dr. Noa Rappaport’s Longevity Consortium paper published in Communications Biology. Original figure credit: Dr. Priyanka Baloni.
Since 1999, the National Institute on Aging (NIA)-funded Longevity Consortium has been exploring the biology behind human aging and longevity. Initially focused on the genetics of human longevity, the consortium has since expanded to study many different facets involved in our lifespans, including genes, proteins, and metabolites, as well as to study age-related diseases and healthy aging. ISB has been an integral part of the Longevity Consortium since 2018, contributing systems biology expertise to integrating and analyzing longevity datasets from several different modalities. Since the consortium’s most recent funding renewal in 2024, ISB researchers, led by Noa Rappaport, PhD, are also involved in research projects to study and predict human longevity among diverse populations and to explore the connections between exceptional longevity and cognitive function.
- Funded by the National Institute on Aging
- Led by Noa Rappaport, PhD
- Key collaborators:
- Lance Pflieger, PhD, Phenome Health
- Robert Moritz, PhD, Kengo Watanabe, PhD, Max Robinson, PhD, and Heeju Noh, PhD, ISB
- Oliver Fiehn, PhD, University of California, Davis
- Nicholas Schork, PhD, The Translational Genomics Research Institute
- Jodi Lapidus, PhD, Oregon Health & Science University
Uncovering the biology of aging and age-related disease
Through work by Longevity Consortium investigators and other research teams, it’s become clear that human longevity and age-related diseases are influenced by many different biological and environmental factors and cannot be completely understood through the lens of any one modality, such as genetics, alone. Additionally, processes that promote longevity may also impact healthy aging and age-related diseases. Understanding the complex network of longevity biology in different contexts remains a difficult but important problem to tackle.
In the Longevity Consortium’s previous funding cycle, Rappaport led the Systems Biology Core at ISB, which developed methods to analyze different forms of data from the consortium’s different research projects to identify biological pathways associated with human longevity.
In the consortium’s current iteration, Rappaport co-leads three components: 1) The Integrative Analysis Core, to develop new methods and perform analyses to integrate disparate datasets from across the consortium’s research projects; 2) Project 1, to study diverse human populations to determine the contexts within which longevity-related factors exhibit associations; and 3) Project 3, to study extreme longevity and its relationship to Alzheimer’s disease and related dementias in diverse human populations.
In previous work with the consortium, ISB researchers contributed to studies of how mouse proteins change when the animals are exposed to interventions that increase their lifespan (namely, calorie restriction, certain drugs, or genetic modifications). By studying large numbers of mouse liver proteins, a tactic known as proteomics, the researchers were able to identify sets of proteins that change in multiple lifespan-increasing interventions. The main changes related to the way the liver cells generate energy. The team is currently conducting analyses on changes in human proteins and metabolism as people age.
In the consortium’s current funding cycle, ISB researchers are also participating in large studies of biological factors that influence human longevity. They’ve used data from existing large cohorts of older people and are following them over time to see which proteins, metabolites and genetic variations correlate with exceptional longevity — living to 94 or older for men and 99 or older for women. They’re now expanding those studies to include more racial and ethnic diversity.
In another project, the researchers are looking at how exceptional longevity appears to protect from cognitive decline. Understanding whether there are common biological factors that drive longevity and protect against Alzheimer’s disease and other forms of age-related dementia could lead to new therapeutic targets for these diseases. In this project, they’re also investigating differences in these biological factors across populations of different racial identities or genetic ancestries.
Citations
- Don J, Schork AJ, Glusman G, Rappaport N, Cummings SR, Duggan D, Raju A, Hellberg KG, Gunn S, Monti S, Perls T, Lapidus J, Goetz LH, Sebastiani P, Schork NJ. The relationship between 11 different polygenic longevity scores, parental lifespan, and disease diagnosis in the UK Biobank. Geroscience. 2024 doi: 10.1007/s11357-024-01107-1.
- Burns AR, Wiedrick J, Feryn A, Maes M, Midha MK, Baxter DH, Morrone SR, Prokop TJ, Kapil C, Hoopmann MR, Kusebauch U, Deutsch EW, Rappaport N, Watanabe K, Moritz RL, Miller RA, Lapidus JA, Orwoll ES. Proteomic changes induced by longevity-promoting interventions in mice. Geroscience. 2024 doi: 10.1007/s11357-023-00917-z.
- Watanabe K, Wilmanski T, Baloni P, Robinson M, Garcia GG, Hoopmann MR, Midha MK, Baxter DH, Maes M, Morrone SR, Crebs KM, Kapil C, Kusebauch U, Wiedrick J, Lapidus J, Pflieger L, Lausted C, Roach JC, Glusman G, Cummings SR, Schork NJ, Price ND, Hood L, Miller RA, Moritz RL, Rappaport N. Lifespan-extending interventions induce consistent patterns of fatty acid oxidation in mouse livers. Commun Biol. 2023 doi: 10.1038/s42003-023-05128-y.
- Sebastiani P, Song Z, Ellis D, Tian Q, Schwaiger-Haber M, Stancliffe E, Lustgarten MS, Funk CC, Baloni P, Yao CH, Joshi S, Marron MM, Gurinovich A, Li M, Leshchyk A, Xiang Q, Andersen SL, Feitosa MF, Ukraintseva S, Soerensen M, Fiehn O, Ordovas JM, Haigis M, Monti S, Barzilai N, Milman S, Ferrucci L, Rappaport N, Patti GJ, Perls TT. A metabolomic signature of the APOE2 allele. Geroscience. 2023 doi: 10.1007/s11357-022-00646-9.

Contact Dr. Noa Rappaport
Principal Scientist
ISB