Jan. 9, 2014
3 Bullets:
- ISB researchers collaborated with Seattle BioMed researchers to identify molecular building blocks required by malaria parasites to build cell membranes.
- Deleting key genes necessary for building cell membranes created a parasite that does not make the host sick and can’t be passed on through the blood.
- Treating mice with modified parasites gave them complete immunity against malaria.
By Dr. Kristian Swearingen
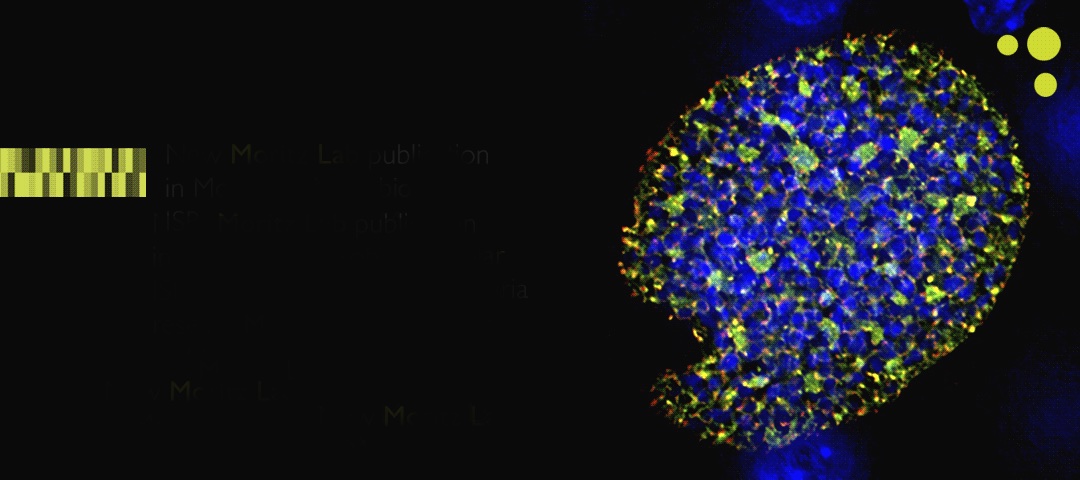
In a study published online in the journal Molecular Microbiology, researchers from ISB and Seattle BioMed identified enzymes critical to the development of cell membranes in Plasmodium yoelii, a malaria-causing parasite that infects rodents. Removing the genes for these enzymes created parasites that could still infect mice but could not complete their life cycle. Infecting mice with these modified parasites acted as a vaccination, giving the mice complete immunity against subsequent infections by fully active malaria parasites.
The disease malaria, caused by parasites of the genus Plasmodium, sickens hundreds of millions of people each year and causes more than half a million deaths. Plasmodium has a complex life cycle: the parasite enters the mammalian host through a mosquito bite and makes its way to the liver, where it transforms and multiplies asexually. The parasite leaves the liver by infecting blood cells, further multiplying until the blood cells burst, causing the disease symptoms of malaria. When infected blood cells are taken up by a feeding mosquito, the parasites mate and make their way to the mosquito’s salivary gland, ready to begin the cycle anew.
Plasmodium parasites scavenge many of the nutrients they need from their hosts. However, certain parts of their life cycle require so much raw material that they need to make it themselves. One such bottle neck is the production of phospholipids, the molecules that make up cell membranes. Plasmodium has its own biochemical machinery to produce the chemical building blocks to make phospholipids. Researchers in the lab of Stefan Kappe at Seattle BioMed used P. yoelii, a species that infects rodents, to study this process. They discovered that two enzymes critical to producing phospholipids were only made by the parasite when it was in the host liver. Deleting the genes for these enzymes created a parasite that could still infect mice and make its way to the liver, but could not mature enough to infect blood cells. These attenuated parasites acted like a vaccine – the infected mice did not get sick because their blood was not infected, and their immune systems were able to build up a response to the parasites that were stuck and ultimately died in their livers. When the immunized mice were then infected with fully active parasites, their immune systems were able to completely fight off the parasites.
Mark Sartain, a lipidomics expert in the lab of Rob Moritz at ISB, applied his expertise to determine specifically what these key enzymes were doing at the chemical level. The Seattle BioMed researchers placed genes for the P. yoelii phospholipid enzymes into bacteria that had had their own phospholipid machinery incapacitated. Mark used quantitative selected reaction monitoring (SRM) mass spectrometry to analyze and quantify the phospholipids created by the bacteria using P. yoelii genes, and learned that the Plasmodium enzymes were indeed producing the chemical building blocks necessary to build phospholipids for cell walls. In-depth analysis of these biochemicals revealed that the Plasmodium genes produce unique combinations and amounts of specific phospholipids. This information provides new insight into phospholipid production in Plasmodium and will aid in the development of new anti-malarial drugs that specifically target this critical biochemical machinery in the parasites.


 isbscience.org/research/the-bug-stops-here-arresting-malaria-parasites-in-the-liver-gives-immunity/
isbscience.org/research/the-bug-stops-here-arresting-malaria-parasites-in-the-liver-gives-immunity/
